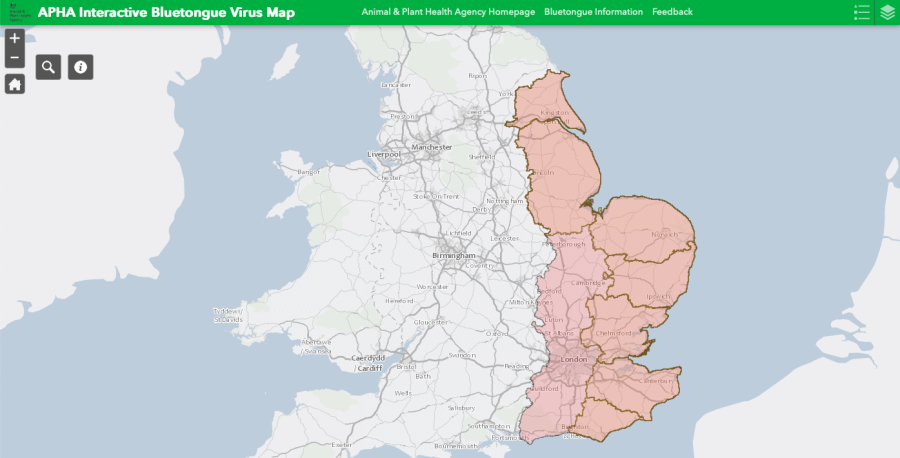
A bluetongue restricted zone (RZ) has been introduced across much of the east of the country, including Essex, Kent and East Sussex, after cases of BTV-3 were identified.
The news has come as a blow to livestock farmers and markets in the affected areas, although, in better news, DEFRA has permitted the use of three unauthorised bluetongue serotype 3 (BTV-3) vaccines within the UK.
The DEFRA announcement pointed out: “Following suspicion of clinical disease in cattle and sheep at premises in East Sussex, Kent, Norfolk and Suffolk, the UK CVO [Chief Veterinary Officer] has confirmed five new cases of BTV-3.”
As a result, The RZ covering Norfolk and Suffolk and Essex, declared on 30 August, was extended to include Kent and East Sussex as well as part of Greater London. An earlier temporary control zone (TCZ) around Faversham was revoked, with the area now included in the restricted zone.
The NFU’s online advice on bluetongue points out that farmers should only move animals within the RZ “where this is absolutely necessary”. It warns: “Any movement carries risk of disease spread.”
The guidance goes on: “Animals may be moved into a market within the zone, but on leaving the market cannot be moved to premises out of the zone. Moves to markets outside the zones are not currently allowed.”
Meanwhile the Secretary of State has granted licences for the use of three ‘unauthorised’ BTV-3 vaccines but has pointed out that they are designed to reduce the effect of bluetongue rather than preventing it.
The NFU explained: “This means they may not prevent your animals from being infected or infectious, but (depending on the vaccine) their claims include reduction or prevention of clinical signs experienced or mortality.” The advice adds: “For this reason, all movement controls and trade restrictions still apply to vaccinated animals.”
The vaccines, Bultavo-3, Bluevac-3 and Sylvazul-3, have been approved in the EU for emergency use and, in the NFU’s words, “could provide a benefit in terms of individual animal health and welfare”.
The manufacturer of Bultavo-3, Boehringer Ingelheim, has said that the vaccine will initially be restricted to high-risk counties in England, including Essex, Kent and East Sussex, “to manage supply and demand”.
It said its inactivated injectable vaccine “has been shown to significantly reduce viraemia and prevent mortality and clinical signs associated with BTV-3 infection”, with immunity in sheep beginning three weeks after a single 1ml subcutaneous dose. In cattle, two 1ml intramuscular doses are required at a three-week interval.
South East Farmer correspondent Alan West described the vaccines as “not perfect but very much better than nothing” and said they would “help no end, depending on availability”.
Alan added that while the initial cases picked up through routine blood testing had not been active or infectious, the recent cases seemed to relate to animals with active symptoms, pointing to the fact that BTV-3 was now circulating.
For more like this, sign up for the FREE South East Farmer e-newsletter here and receive all the latest farming news, reviews and insight straight to your inbox.